 Your current location: Home > Technical Support > Popular Science Column > Fermion's 100 Q...
Your current location: Home > Technical Support > Popular Science Column > Fermion's 100 Q... 7. What is the ultimate vacuum that various materials can achieve?
This question covers a wide range, because the ultimate vacuum that each material can achieve is closely related to the environment and state of the material, and cannot be simply discussed based on the material.
Take a simple example, water. The figure below shows the saturated vapor pressure of water at different temperatures. (Saturated vapor pressure refers to the pressure when the gas and liquid are in two-phase equilibrium. This concept has very important applications in the vacuum field, including in the field of molecular beam epitaxy)
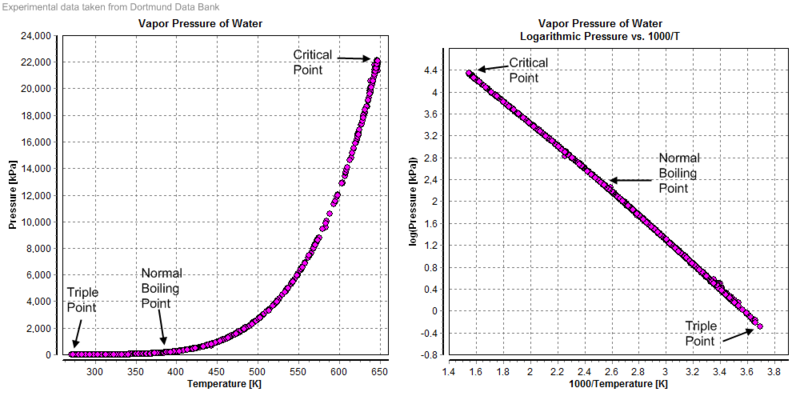
From the above data, we can see that the vapor pressure of water increases exponentially with temperature. We also use this principle to bake (Bakeout), so that the components inside the vacuum release the adsorbed water vapor at high temperature, thereby increasing the ultimate vacuum of the system after cooling down.
If you need to query the saturated vapor pressure of a specific material, you can use a more common search engine; the attachment contains an empirical calculation formula for the saturated vapor pressure of a metal material, which can give the saturated vapor pressure of many common materials: log(p/atm) = A + B/T + C*log(T) + D/T3
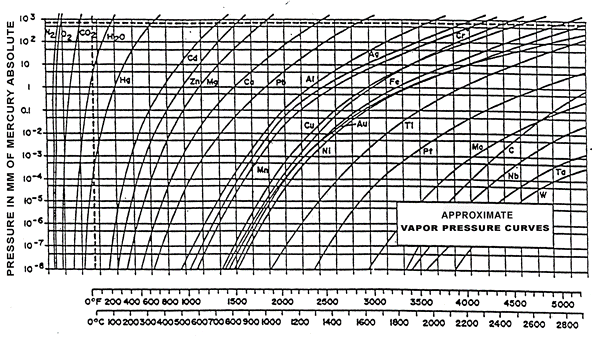
8. What are the commonly used vacuum metal and non-metal materials, and how to choose them?
Commonly used metal materials
● Stainless steel 304, 316, 316L, 316 LN
The most common material, structural parts below 600℃, simple processing, and controllable cost.
● Oxygen-free copper, beryllium bronze, phosphor bronze
Oxygen-free copper is generally used for low-temperature heat conduction or gaskets and other materials. It can work from extremely low temperatures to about 500℃, but it is not suitable as a structural part in high temperature areas because its strength is greatly reduced.
Beryllium bronze and phosphor bronze are characterized by great hardness and wear resistance, so they can be used as bearings or wear-resistant parts inside vacuum.
Beryllium bronze is also used in the manufacture of many shrapnel and pins due to its elastic characteristics.
●Tantalum, molybdenum, tungsten, titanium
These are all high-temperature resistant metals. In terms of high-temperature resistance, the arrangement from low to high is titanium, molybdenum, tantalum, tungsten
In terms of processing difficulty, tungsten has the highest hardness and difficulty. Molybdenum machined parts are relatively brittle. The processing of tantalum is similar to that of stainless steel. Titanium will experience knife sticking as the temperature rises during processing.
In terms of corrosion resistance, titanium forms a dense protective layer on the surface and can be used in high-temperature oxidation environments.
Molybdenum will form molybdenum oxide above 500°C in the presence of oxygen, and molybdenum dioxide will biodegrade at high temperatures, which will cause the original parts made of molybdenum to corrode continuously in high-temperature oxidation environments.
Common non-metallic materials
● Alumina ceramics
Alumina ceramics are widely used in vacuum environments because of their simple processing, especially the low cost of batch processing. They are generally used as insulating gaskets or high-temperature thermal insulation materials.
● Polytetrafluoro
Polytetrafluoroethylene has a very stable property and is self-lubricating. Therefore, it is often used as lubricating and insulating components inside a vacuum. The maximum operating temperature of polytetrafluoroethylene can reach about 200°C, so it can meet the baking requirements of ultra-high vacuum.
● PEEK
Polyetheretherketone (PEEK) resin is a special engineering plastic with excellent performance. Compared with other special engineering plastics, it has more significant advantages, such as high temperature resistance of 260 degrees, excellent mechanical properties, good self-lubrication, chemical corrosion resistance, flame retardant, peeling resistance, wear resistance, not resistant to strong nitric acid, concentrated sulfuric acid, radiation resistance, and super strong mechanical properties. It can be used in high-end machinery, nuclear engineering, aviation and other technologies.
Compared with polytetrafluoroethylene, PEEK can be modified by doping to obtain many sub-materials with different properties, such as friction resistance, corrosion resistance, heat insulation, etc. The following is a property comparison table of common engineering plastics.
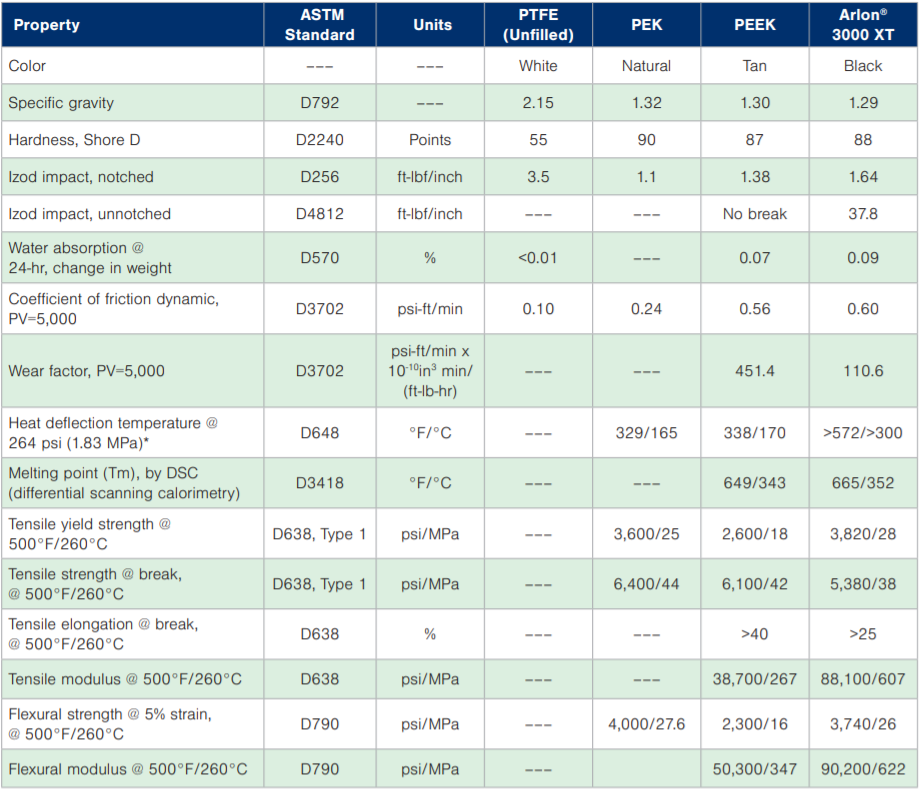
Related links: (https://www.gtweed.com/materials/thermoplastics-and-composites/peek-vs-pek-vs-ptfe/)
9. Why is molybdenum disulfide applied to the meshing of the sample rack gears? Does it have any effect on the vacuum?
Gear meshing If the parts are not properly lubricated, dry friction will occur, causing wear on the gears, possibly resulting in gear surface damage, transmission failure or even jamming. Short-term high-speed friction may even generate a large amount of heat, affecting the cooling effect of the sample rack. Pure molybdenum disulfide is a black solid, layered crystal. Each atomic monolayer of the material is only bound by van der Waals force, which is weak and easy to slip, so it has good lubricity and can be used as a solid lubricant. Its melting point is 2375℃, and it sublimates at 450℃ in an inert atmosphere. , so pure molybdenum disulfide will not affect the vacuum degree when used in a vacuum.
The "molybdenum disulfide" actually used as a lubricant is usually added with other greases, surfactants, etc. Additives. According to the content of MoS2, it can be divided into
For example, Dow Corning molybdenum disulfide, which is commonly used for screw lubrication, contains mineral oil, activator and other additives in addition to molybdenum disulfide powder. Mineral oil The saturated vapor pressure at room temperature is high and the boiling point is high, making it difficult to remove quickly by baking, so it cannot be used in a vacuum.

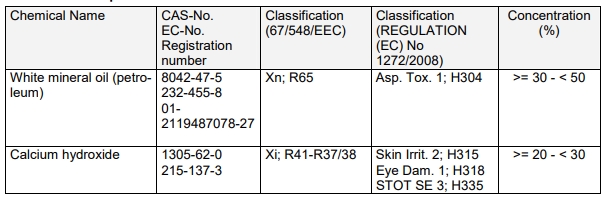
For quick-drying molybdenum disulfide spray, in addition to molybdenum disulfide, graphite and polytitanate butylene, other They are all volatile organic substances. Therefore, after use in the atmosphere, the organic substances will evaporate quickly, and the only residues are solid lubricants such as molybdenum disulfide and graphite. These solid lubricants have high boiling points and low vapor pressures and will not affect the vacuum. .

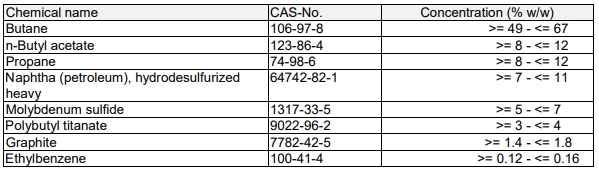
In summary, whether molybdenum disulfide affects vacuum depends on the type of additive. Greases with high boiling point and high vapor pressure must not be used in a vacuum, while those that are volatile and leave no residue can be used in a vacuum.


