Your current location: Home > Technical Support > Popular Science Column > Fermion's 100 Q...
Your current location: Home > Technical Support > Popular Science Column > Fermion's 100 Q... 10. Why can't the ion pump be turned on under rough vacuum?
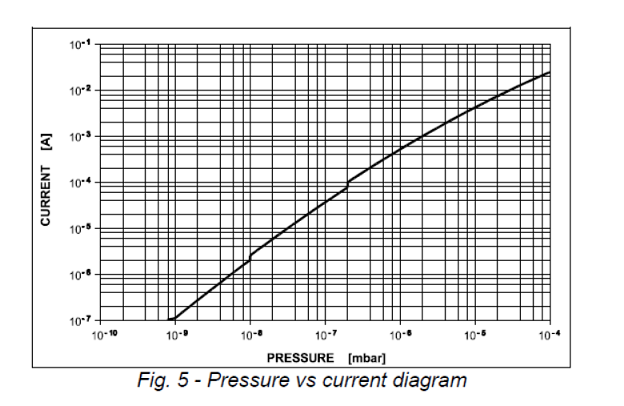
This question depends on the specific vacuum range. If it is in the vacuum degree of glow discharge, such as ~10Pa, the gas will be broken down by the high voltage of the ion pump. At this time, the ion current is very large, which will far exceed the load of the ion pump power supply. Under normal circumstances, the ion pump power supply will automatically cut off the output and alarm.
If the vacuum degree is higher and meets the startup requirements of the ion pump, we must also pay attention to the anode life of the ion pump. Under relatively poor vacuum conditions, the discharge ion flow is actually maintained by corroding the anode. The actual life of the pump is related to the working current.
Taking the Agilent ion pump (Vaclon Plus 20) as an example, we can see that the definition of life is actually related to vacuum. Generally, under a vacuum of E-6 mbar, the life of this pump is 80,000 hours (about a little more than 9 years of continuous operation).
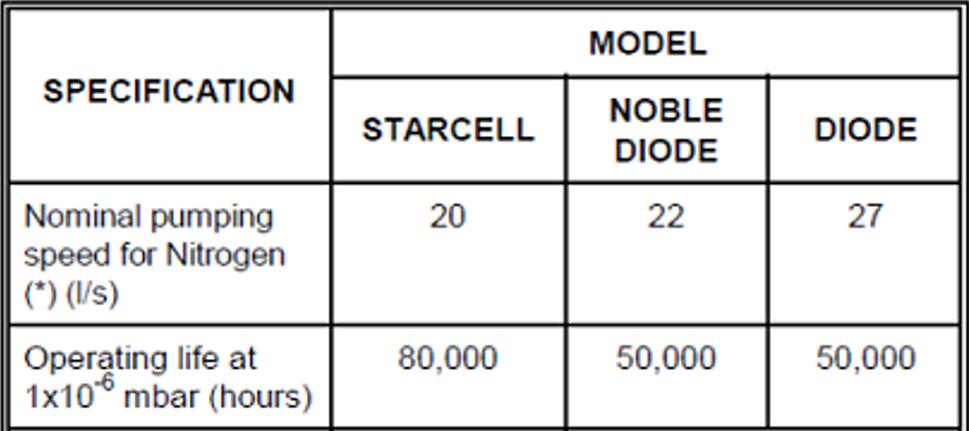
From an exponential point of view, the current and vacuum degree are basically linearly related, so if the vacuum is one order of magnitude different, the life span will also be reduced by one order of magnitude. Simply converted, under the vacuum condition of E-5 mbar, the life span of the same pump set is less than one year.
11. Will the vacuum degree be better if a layer of adsorption material is coated on the inner surface of the vacuum chamber?
Yes. The fresh surfaces of many metal elements can be used as vacuum adsorption materials, such as Ti, Ce, etc. Ti pump is a widely used vacuum pump, which realizes vacuum adsorption by evaporating fresh titanium onto the cavity wall through titanium rod.
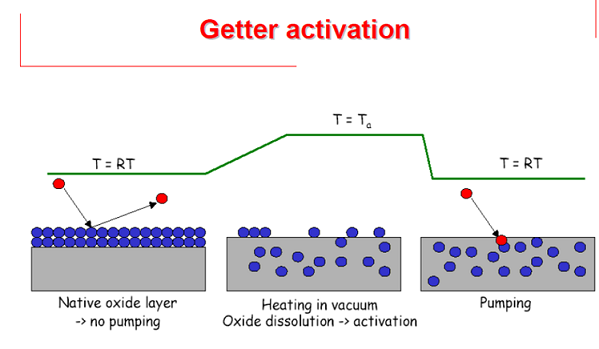
The linear pipeline of modern synchrotron radiation basically uses NEG (Non-Evaporable Getter) material sputtered on the inner wall to achieve ultra-high vacuum, while reducing the secondary electrons caused by electrons hitting the cavity wall and avoiding contamination of optical components. NEG is a popular technology at present, mainly zirconium iron vanadium alloy material, which does not need to be evaporated. It is activated by heating in a vacuum, and the gas adsorbed on the surface is transferred to the body (except hydrogen) to repeatedly obtain a fresh surface and realize vacuum adsorption.

12. What is the purpose of activating the NEG pump?
The NEG pump, a non-evaporable getter pump, can be simply regarded as an adsorption pump composed of a very active metal tablet. As the amount of adsorption increases, the surface activity of the getter will continue to decrease, and the pumping speed will also continue to decrease. At this time, the NEG pump needs to be reactivated.
The activation operation of the NEG pump is very simple. Heating allows various gases (except hydrogen) adsorbed on the surface of the getter to diffuse into the body under high temperature, thereby obtaining a clean surface and regaining a huge adsorption capacity; while hydrogen is released from the surface into the vacuum chamber and pumped away by other pumps. Therefore, the activation life of NEG we generally refer to other gases except hydrogen. Even if the other gases in the NEG material are saturated, it can still be used as a pump to extract hydrogen.
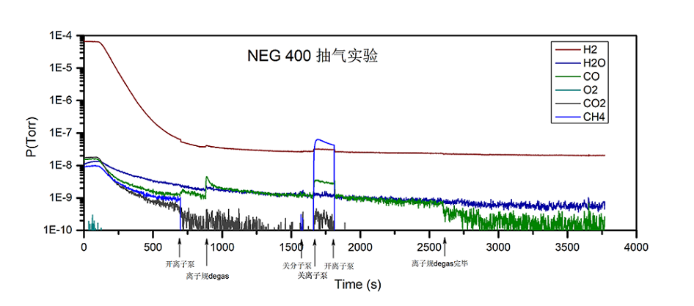
In addition, NEG cannot extract inert gas and CH4. It is likely to release a certain amount of CH4 when NEG is used. The problem of CH4 is more complicated. The figure below shows the changes in various gas components during the use of our NEG400 with molecular pumps and ion pumps. It can be seen that when the molecular pump and ion pump are turned off, CH4 has a significant increase and greatly exceeds the background CH4 value, while it has little effect on H2 and H2O, and has good adsorption performance.