 Your current location: Home > Technical Support > Popular Science Column > Fermion's 100 Q...
Your current location: Home > Technical Support > Popular Science Column > Fermion's 100 Q... 4. When vacuuming at high temperature, can the vacuum index be better after the cavity cools down?
The ultra-high vacuum system (mainly metal sealing) is mainly based on the cavity made of SS316 (or SS304) as the carrier, and is equipped with components with different functions (all of which are made of materials that meet the requirements of ultra-high vacuum low degassing rate).
The inner surface of the vacuum chamber is generally polished (mechanical polishing, electrochemical polishing, etc.) to achieve a smoother and denser surface. After the vacuum system is exposed to the atmosphere (vent), not only the inner surface of the chamber, but all the components in the chamber will adsorb gas (mostly water vapor). If it is not baked (i.e. at room temperature), the adsorbed water vapor will slowly desorb, and this process will last for a very long time.
The baking process will accelerate the desorption rate of water vapor and greatly shorten the time the system enters ultra-high vacuum (E-10 mbar level).
Below is a comparison chart of baking and not baking.
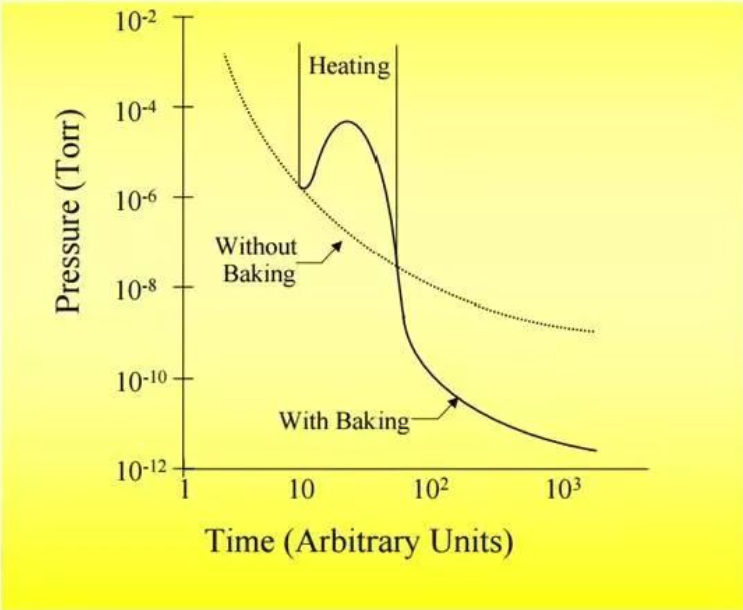
After the system is installed (or after the vacuum is restored), it is necessary to start baking when the vacuum is better than E-5 mbar.
Ultra-high vacuum systems are equipped with different electronic components. The baking temperature is generally set to 120 degrees Celsius (exceeding the vaporization temperature of water). The baking time is set according to the complexity of the system, generally not less than 48 hours; during the baking process, the ion gauge needs to be degassed intermittently (for example, every 10 hours).
The baking effect is generally judged by 100 times the expected ultimate vacuum. For example, if the ultimate vacuum is 5E-10 mbar, the baking can be stopped when the vacuum reaches 2E-8 mbar under 120 degrees baking conditions; if the vacuum system is more complex, it can be maintained at the E-8mbar level for a longer time.
5. What vacuum degree requirements can be reached before the temperature can be reduced, and why?
Different industries and environments have different requirements, which depends entirely on the actual use environment. Generally speaking, the influence of vacuum on low temperature mainly lies in the following aspects: The influence of low temperature condensation on the test We must realize that even on the surface of E-7 mbar, it only takes 1s to adsorb a layer of gas molecules at room temperature. As the temperature decreases, more and faster gas molecules will be adsorbed on the surface, especially water, oxygen, nitrogen, etc., which are common in the air, which are easily adsorbed to the sample surface at low temperatures.
We need to evaluate the impact of this adsorption on the test. On the one hand, low-temperature adsorption will affect the sensitivity of surface testing. For example, ARPES, XPS, etc. are only sensitive to the surface at a few to dozens of atomic layers. Once the adsorbed gas molecules are very thick, they will affect our test. In addition, the analysis of gas adsorbed on the surface will also affect the properties of the surface. For example, after oxygen is adsorbed on the metal surface, it is easy to undergo oxidation reactions, thereby changing the properties of the material. Some of our equipment also uses this to complete low-temperature adsorption and catalysis.
From this point of view, for surface-sensitive experiments, it is generally recommended to start cooling the vacuum to at least E-9 mbar. However, for some non-surface testing methods, such as spectroscopy, XRD, etc., as long as the surface is prevented from freezing, the vacuum can generally reach E-4 mbar.
Effect of vacuum on thermal insulation
Gas molecules can act as a heat-conducting medium, so it is necessary to evaluate the relationship between vacuum and extremely low temperature. Generally, the temperature of liquid helium will be particularly obvious because the cooling capacity of liquid helium is relatively small. In order to achieve extremely low temperatures, it is necessary to reduce additional heat losses. From this point of view, it is generally sufficient for the vacuum to reach E-3 mbar.
6. What are ionization absorption, physical adsorption, and chemical adsorption? Which one can achieve a better vacuum index?
Ionization inhalation is usually related to the working principle of ion pumps. Under high pressure conditions, the gas is ionized. The gas ions generated after ionization are bombarded to the electrode and buried under the action of electric and magnetic fields, producing the effect of "inhalation".
Physical adsorption is usually related to the working principle of condensation pumps. It refers to the combination of gas molecules and the surface through weak interaction forces (van der Waals force, electrostatic effect, etc.) to achieve the effect of "inhalation". Because the force of physical adsorption is weak, it is easy to desorb when the temperature is increased, so the surface needs to have a lower temperature to produce the adsorption effect.
Chemical adsorption is usually related to the working principle of room temperature adsorption pumps such as adsorbent pumps and titanium sublimation pumps. It refers to the "inhalation" effect achieved by the strong interaction (chemical bond) between gas molecules and the surface. Because the chemical adsorption force is strong, the gas adsorbed at room temperature will not desorb, so it can work at room temperature and produce the adsorption effect.
Ionization inhalation, physical adsorption and chemical adsorption have different pumping speeds for different gases:
1. Ionization inhalation depends on the ionization cross section of the gas molecules. The larger the ionization cross section, the easier the gas is ionized, and the better the "inhalation" effect. For example, the pumping speed of an ion pump for an inert gas is very low because the ionization cross section of an inert gas is small.
2. Physical adsorption depends on the magnitude of the force between the gas molecules and the surface of action. Usually, the magnitude of this force is related to the boiling point of the gas, so the boiling point of the gas can be used to determine the difficulty of its physical adsorption. The lower the boiling point of the gas, the lower the temperature of the adsorption surface needs to be in order to achieve a better adsorption effect. For example, at liquid nitrogen temperature, gases such as hydrogen do not adsorb, and the liquid nitrogen condensation pump has no pumping speed for hydrogen.
3. Chemical adsorption depends on the chemical activity of the adsorbent and the strength of the interaction between the adsorbed gas and the adsorbent. The adsorbent used in vacuum needs to meet the characteristics of easy repeated activation, high chemical activity, and non-volatile. Active transition metals and their alloys are good choices for adsorbents. For example, metal or alloy surfaces usually have good storage capacity for small molecular gases such as hydrogen, and their "absorption" capacity for hydrogen is also stronger.
Vacuum indicators are usually measured by vacuum gauges, so for different gases, the level of vacuum indicators is also related to the measurement principle of the vacuum gauge. For example, the ion gauge obtains the vacuum degree by measuring the microcurrent through thermal electron ionization, and the measurement accuracy is different for gases with different ionization cross sections. Therefore, it is very likely that a certain vacuum pump has a high pumping speed for a certain gas, but because the vacuum gauge does not measure this gas accurately, the apparent vacuum degree does not change.
In order to better reflect the vacuum index, it is necessary to use RGA to observe the gas content at each mass number. This can more objectively explain which gas can achieve better vacuum indicators for ionization absorption, physical adsorption and chemical adsorption.
Usually, the pumping speed index of a vacuum pump is based on the pumping speed of N2.The vacuum gauge is also calibrated based on the measurement of N2. Taking N2 as the pumped gas, whether it is an ion pump, condensation pump or adsorbent pump, the higher the pumping speed, the better the vacuum index can be achieved.


